Annie Jenney, NP/PA, bridges the gap between Fenway Health’s Sexual Health Clinic and The Fenway Institute’s research programs. Recently, Annie cared for a patient who came in with a lesion. She made sure the patient received the right diagnostic care, and then personally introduced them to the research team. That patient became the very first participant in our new Point-of-Care Lesion Diagnostic Study.
Thanks to Annie, the patient received high-quality, low-barrier care, and also had the opportunity to take part in research—turning a stressful medical situation into something positive. Not only did the patient get support and answers, they were also compensated for their time, contributing to science that will improve care for others.
“My role as a Sexual Health and TFI Research PA allows me to do a little more for my patients,” Annie explained. “Because we’re a walk-in, low-barrier clinic, we often see people who might not be able to access care otherwise. Our Health Navigators make patients feel at ease from the moment they arrive, so by the time I meet them, they’re ready to share what’s really bothering them. It’s meaningful to offer them the chance to help move science forward while also being compensated—it can bring a silver lining to an otherwise tough day.”
Working with The Fenway Institute research staff makes this kind of seamless integration possible. As Annie described, they’re able to connect patients who are already here for care with studies that fit their needs. The Point-of-Care Lesion Study, in particular, could be a game-changer. It’s designed to help patients get an accurate diagnosis for conditions like HSV-1 and 2, mpox, varicella (chickenpox and shingles), and syphilis—sometimes in as little as 20 minutes.
Dr. Taimur Khan, Associate Medical Research Director and Primary Care Physician, reflected: “I was incredibly proud of how our team came together in this moment. We provided compassionate, timely care while also connecting the patient to research. It’s a great example of how Fenway combines low-barrier access with innovation—supporting patients while advancing science.”
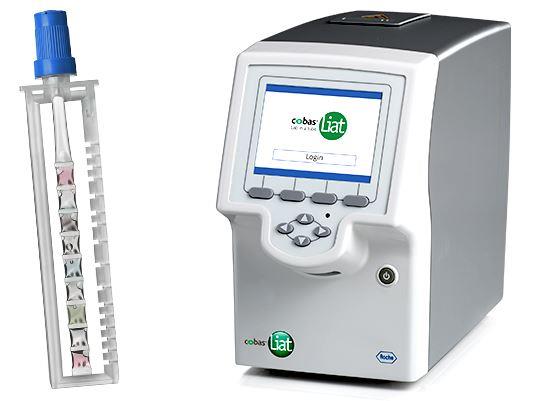
About the Lesion Study
The Lesion Study, funded by Roche Diagnostics, is helping validate a new test on the Cobas Liat machine. Once approved, it will allow providers to swab a lesion and get results in minutes instead of waiting days for lab results. Fenway’s role is to enroll 50 participants, each compensated $100, to compare these rapid test results against current FDA-approved methods. While results from the research swab aren’t shared with patients, a standard swab can also be collected for clinical testing if needed.